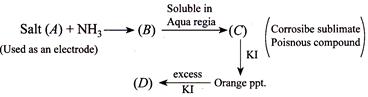
 The compound and are, respectively,
The compound and are, respectively,
A) \[{{H}_{2}}N.HgCl,\,HgC{{l}_{2}}\]
B) \[{{H}_{2}}N.HgC{{l}_{2}},{{H}_{2}}Hg{{I}_{4}}\]
C) \[{{H}_{2}}N.HgCl+Hg,\,{{K}_{2}}[Hg{{I}_{4}}]\]
D) \[[Hg{{(N{{H}_{3}})}_{2}}]Cl,\,\,Hg{{I}_{4}}\]
Correct Answer: C
Solution :
[c] Salt [a] (\[H{{g}_{2}}C{{l}_{2}},\]calome1'ionic compound)is blackened by \[N{{H}_{3}}\]. (i) \[HgC{{l}_{2}}(A)+2N{{H}_{3}}\xrightarrow{{}}\underset{\begin{smallmatrix} (B) \\ (Black) \end{smallmatrix}}{\mathop{[{{H}_{2}}N.HgCl+Hg]}}\,+N{{H}_{4}}Cl\](ii)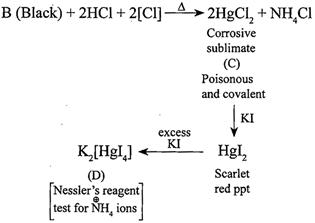
You need to login to perform this action.
You will be redirected in
3 sec
