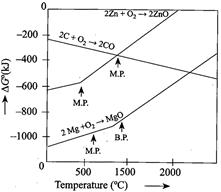
 Which of the following statements is wrong?
Which of the following statements is wrong?
A) At \[1000{}^\circ C,\] \[Zn\]and \[C\]have equal affinity for \[{{O}_{2}}\]
B) At \[1000{}^\circ C,\] \[{{\Delta }_{r}}{{G}^{\Theta }}\] for \[(ZnO+C\xrightarrow{{}}Zn+CO)\]is negative.
C) Above \[1000{}^\circ C,\] reaction \[(ZnO+C\xrightarrow{{}}Zn+CO)\] is spontaneous.
D) At \[1100{}^\circ C,\] \[{{\Delta }_{r}}{{G}^{\Theta }}\] for \[(ZnO+Mg\xrightarrow{{}}MgO+Zn)\] has highest negative value.
Correct Answer: B
Solution :
[b] (1) Statement (1) is CORRECT At \[\tilde{\ }1000{}^\circ C,\]lines \[(C,CO)\] and \[(Z,ZnO)\] intersect; at this temperature zinc and carbon have equal affinity for oxygen. (2) Statemenet (2) INCORRECT At \[\tilde{\ }1000{}^\circ C,\]since \[Zn\]and \[C\] have equal affinity for \[{{O}_{2}},\] \[{{\Delta }_{r}}{{G}^{\Theta }}\]of the reaction is zero. \[ZnO+C\xrightarrow{{}}Zn+CO\] (3) Statement (3) is CORRECT To make the reaction \[ZnO+C\xrightarrow{{}}Zn+CO\] to proceed in forward direction, temperature should be greater than \[1100{}^\circ C\] (4) Statement (4) is CORRECT At \[1100{}^\circ C,\] \[{{\Delta }_{r}}{{G}^{\Theta }}\]for the reaction \[ZnO+Mg\xrightarrow{{}}MgO+Zn\]has highest negative value, hence this reaction will be spontaneous to a maximum. CORRECT statement:- \[{{\Delta }_{r}}{{G}^{\Theta }}=0\]You need to login to perform this action.
You will be redirected in
3 sec
