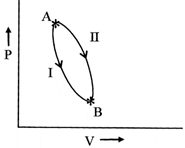
 As per path -I, \[\Delta q=-400\,cal\] and \[\Delta W=14cal\] As per path-II, \[\Delta q=-48cal\]. Therefore work done, \[\Delta W\] in path -(II) is-
As per path -I, \[\Delta q=-400\,cal\] and \[\Delta W=14cal\] As per path-II, \[\Delta q=-48cal\]. Therefore work done, \[\Delta W\] in path -(II) is-
A) \[-338\,cals\]
B) \[-366\,cals\]
C) \[-434\text{ }cals\]
D) \[-462\text{ }cals\]
Correct Answer: A
Solution :
[a] For path - I \[\Delta {{E}_{1}}=\Delta q+\Delta W=-400+14\] \[=-386\,cals\] For path -II \[\Delta {{E}_{II}}=\Delta {{E}_{I}}=-48+\Delta W\] \[\therefore \text{ }\Delta W=-386+48=-338cal\]You need to login to perform this action.
You will be redirected in
3 sec
