 What is the electrochemical equivalent of Cu?
What is the electrochemical equivalent of Cu?
A) m/2
B) m/3
C) m/4
D) \[\frac{m}{63.5}\]
Correct Answer: B
Solution :
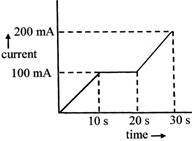
[b] Total charge passed in 30 sec. \[=1\times 100\times 10+100\times 10+100\times 10+\frac{1}{2}\] \[\times 100\times 10\] \[=3\times 100\times 10mA\times s\] \[=3000\times {{10}^{-3}}amp\text{ }s=3C\] W=ZQ \[\therefore Z=\frac{W}{Q}=\frac{m}{3}\]You need to login to perform this action.
You will be redirected in
3 sec
