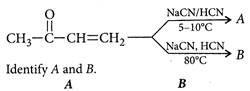
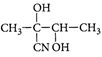
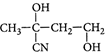
A)
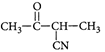
B)
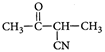
C)
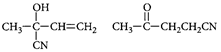
D) None of these.
Correct Answer: C
Solution :
[c] : The concept is based on thermo- dynamically and kinetically controlled reaction. When the temperature is lower, reaction is irreversible and kinetically controlled. So, due to more polarity ofYou need to login to perform this action.
You will be redirected in
3 sec
