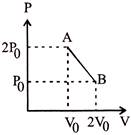
A) \[\frac{9{{P}_{0}}{{V}_{0}}}{2nR}\]
B) \[\frac{9{{P}_{0}}{{V}_{0}}}{nR}\]
C) \[\frac{9{{P}_{0}}{{V}_{0}}}{4nR}\]
D) \[\frac{3{{P}_{0}}{{V}_{0}}}{2nR}\]
Correct Answer: C
Solution :
The equation for the line is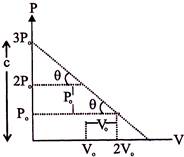 \[P=\frac{-{{P}_{0}}}{{{V}_{0}}}V+3P\,[slope\,\,=\frac{-{{P}_{0}}}{{{V}_{0}}},\,c=3{{P}_{0}}]\] \[P{{V}_{0}}+{{P}_{0}}V=3{{P}_{0}}{{V}_{0}}\] ?.(i) But \[PV=nRT\] \[\therefore \,\,P=\frac{nRT}{V}\] ....(ii) From (i) and (ii) \[\frac{nRT}{V}{{V}_{0}}+{{P}_{0}}V=3{{P}_{0}}{{V}_{0}}\] \[\therefore \,\,nRT\,\,{{V}_{0}}+{{P}_{0}}{{V}^{2}}=3{{P}_{0}}{{V}_{0}}\] ?.(iii) For temperature to be maximum \[\frac{dT}{dV}=0\] Differentiating e.q. (iii) by 'V? we get \[nR{{V}_{0}}\frac{dT}{dV}+{{P}_{0}}(2V)=3{{P}_{0}}{{V}_{0}}\] \[\therefore \,\,nR{{V}_{0}}\frac{dT}{dV}=3{{P}_{0}}{{V}_{0}}-2{{P}_{0}}V\] \[\frac{dT}{dV}=\frac{3{{P}_{0}}{{V}_{0}}-2{{P}_{0}}V}{nR{{V}_{0}}}=0\] \[V=\frac{3{{V}_{0}}}{2}\] \[\therefore \,\,P=\frac{3{{P}_{0}}}{2}\] [From (i)] \[\therefore \,\,{{T}_{\max }}=\frac{9{{P}_{o}}{{V}_{o}}}{4nR}\] [From (iii)]
\[P=\frac{-{{P}_{0}}}{{{V}_{0}}}V+3P\,[slope\,\,=\frac{-{{P}_{0}}}{{{V}_{0}}},\,c=3{{P}_{0}}]\] \[P{{V}_{0}}+{{P}_{0}}V=3{{P}_{0}}{{V}_{0}}\] ?.(i) But \[PV=nRT\] \[\therefore \,\,P=\frac{nRT}{V}\] ....(ii) From (i) and (ii) \[\frac{nRT}{V}{{V}_{0}}+{{P}_{0}}V=3{{P}_{0}}{{V}_{0}}\] \[\therefore \,\,nRT\,\,{{V}_{0}}+{{P}_{0}}{{V}^{2}}=3{{P}_{0}}{{V}_{0}}\] ?.(iii) For temperature to be maximum \[\frac{dT}{dV}=0\] Differentiating e.q. (iii) by 'V? we get \[nR{{V}_{0}}\frac{dT}{dV}+{{P}_{0}}(2V)=3{{P}_{0}}{{V}_{0}}\] \[\therefore \,\,nR{{V}_{0}}\frac{dT}{dV}=3{{P}_{0}}{{V}_{0}}-2{{P}_{0}}V\] \[\frac{dT}{dV}=\frac{3{{P}_{0}}{{V}_{0}}-2{{P}_{0}}V}{nR{{V}_{0}}}=0\] \[V=\frac{3{{V}_{0}}}{2}\] \[\therefore \,\,P=\frac{3{{P}_{0}}}{2}\] [From (i)] \[\therefore \,\,{{T}_{\max }}=\frac{9{{P}_{o}}{{V}_{o}}}{4nR}\] [From (iii)]
You need to login to perform this action.
You will be redirected in
3 sec
