 The compound (X) is
The compound (X) is
A) \[Mg{{I}_{2}}\]
B) \[MgB{{r}_{2}}\]
C) \[Ca{{I}_{2}}\]
D) \[CaB{{r}_{2}}\]
Correct Answer: B
Solution :
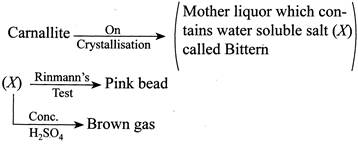
[b] Camallite is dissolved in \[{{H}_{2}}O\] and \[KCl\] and \[MgC{{l}_{2}}\] is are crystallised out. The mother liquor contains \[0.25%\]of bromine as \[MgB{{r}_{2}}\] and is known as Bitter which is treated with \[C{{l}_{2}}(g)\] (g) to give \[B{{r}_{2}}(I)\]. \[MgB{{r}_{2}}+C{{l}_{2}}\xrightarrow{{}}MgC{{l}_{2}}+B{{r}_{2}}\] Thus camallite contains \[MgB{{r}_{2}},\] as impurity. On crystallisation \[MgB{{r}_{2}},\] remains in mother liquor. (i) \[MgB{{r}_{2}}\,\,(X)\] gives in cobalt nitrate test (Rinmann's test), a pink mass. \[2Co{{(N{{O}_{3}})}_{2}}\xrightarrow{\Delta }2CoO+4N{{O}_{2}}+{{O}_{2}}\] \[CoO+MgO\xrightarrow{{}}CaO.MgO\,\,\,(Pink\,mass)\] (ii) \[\underset{(X)}{\mathop{MgB{{r}_{2}}}}\,+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}MgS{{O}_{4}}+2HBr\] \[2HBr+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}2{{H}_{2}}O+S{{O}_{2}}+B{{r}_{2}}\,\,(Brown\,\,fumes)\]You need to login to perform this action.
You will be redirected in
3 sec
