A) oxygen gives colour to it.
B) charge transfer when oxygen gives its electron to Mn making it Mn (+VI) hence, coloured.
C) charge transfer when Mn gives its electron to oxygen.
D) none of the above is correct.
Correct Answer: B
Solution :
[b]: The colour arise by charge transfer. In \[MnO_{4}^{-}\], an electron is momentarily transferred from oxygen to the metal and thus oxygen changes from \[{{O}^{2-}}\] to \[{{O}^{-}}\] and Mn from (+7) to (+6).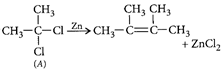
You need to login to perform this action.
You will be redirected in
3 sec
