A) \[[N{{F}_{3}},NO_{3}^{-}] and \,[B{{F}_{3}},{{H}_{3}}{{O}^{+}}]\]
B) \[[N{{F}_{3}},HN_{3}^{{}}] and \,[NO_{3}^{-},B{{F}_{3}}]\]
C) \[[N{{F}_{3}},{{H}_{3}}O_{{}}^{+}] and \,[NO_{3}^{-},B{{F}_{3}}]\]
D) \[[N{{F}_{3}},{{H}_{3}}O_{{}}^{+}] and \,[HN_{3}^{{}},B{{F}_{3}}]\]
Correct Answer: C
Solution :
[c] : I so structural pairs have similar structures.| \[N{{F}_{3}}\] | Triangular pyramidal | |
| \[NO_{3}^{-}\] | 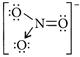 |
Triangular planar |
| \[B{{F}_{3}}\] | Triangular planar | |
| \[{{H}_{3}}{{O}^{+}}\] | Triangular pyramidal | |
| \[H{{N}_{3}}\] | Linear |
You need to login to perform this action.
You will be redirected in
3 sec
