A) \[{{\lambda }_{3}}={{\lambda }_{1}}+{{\lambda }_{2}}\]
B) \[{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
C) \[{{\lambda }_{1}}+{{\lambda }_{2}}+{{\lambda }_{3}}=0\]
D) \[\lambda _{3}^{2}=\lambda _{1}^{2}+\lambda _{2}^{2}\]
Correct Answer: B
Solution :
Idea This question is based on the energy levels of an atom and the transition of the electrons between them.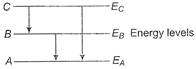 From the figure, \[({{E}_{A}}-{{E}_{B}})+({{E}_{B}}-{{E}_{C}})=({{E}_{C}}-{{E}_{A}})\] \[\Rightarrow \]\[\frac{hc}{{{\lambda }_{1}}}+\frac{hc}{{{\lambda }_{2}}}=\frac{hc}{{{\lambda }_{3}}}\] \[\Rightarrow \]\[{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\] TEST Edge Similar questions based on the energy levels of hydrogen atom are normally asked in JEE Main.
From the figure, \[({{E}_{A}}-{{E}_{B}})+({{E}_{B}}-{{E}_{C}})=({{E}_{C}}-{{E}_{A}})\] \[\Rightarrow \]\[\frac{hc}{{{\lambda }_{1}}}+\frac{hc}{{{\lambda }_{2}}}=\frac{hc}{{{\lambda }_{3}}}\] \[\Rightarrow \]\[{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\] TEST Edge Similar questions based on the energy levels of hydrogen atom are normally asked in JEE Main.
You need to login to perform this action.
You will be redirected in
3 sec
