A) \[2\sqrt{3}\overset{{}^\circ }{\mathop{\Alpha }}\,\]
B) \[\sqrt{3}\overset{{}^\circ }{\mathop{\Alpha }}\,\]
C) \[4\sqrt{3}\overset{{}^\circ }{\mathop{\Alpha }}\,\]
D) \[\frac{1}{\sqrt{3}}\overset{{}^\circ }{\mathop{\Alpha }}\,\]
Correct Answer: A
Solution :
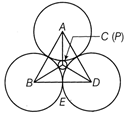 [C = centeroid] \[\frac{BE}{BC}=\cos 30{}^\circ =\frac{\sqrt{3}}{2},\]\[\frac{BE}{2}=\frac{\sqrt{3}}{2}\Rightarrow BE=\sqrt{3}\] Distance between the centres of two Q atoms \[=2\times BE=2\times \sqrt{3}=2\sqrt{3}\overset{{}^\circ }{\mathop{\Alpha }}\,\]
[C = centeroid] \[\frac{BE}{BC}=\cos 30{}^\circ =\frac{\sqrt{3}}{2},\]\[\frac{BE}{2}=\frac{\sqrt{3}}{2}\Rightarrow BE=\sqrt{3}\] Distance between the centres of two Q atoms \[=2\times BE=2\times \sqrt{3}=2\sqrt{3}\overset{{}^\circ }{\mathop{\Alpha }}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
