A) 5, acidic
B) 7, basic
C) 6, neutral
D) 7, acidic
Correct Answer: D
Solution :
This problem includes conceptual mixing of electrophilic substitution reaction, degree of unsaturation and acidic/basic nature. Electrophilic Substitution Reaction Phenol undergo electrophilic substitution reaction on reaction with a mixture of cone. \[HN{{O}_{3}}\]and cone. \[{{H}_{2}}S{{O}_{4}}\](known as nitrating mixture). This reaction produces nitro compound and on successive nitration it produces trinitro phenol. conc.\[HN{{O}_{3}}+conc.{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{\overset{+}{\mathop{O}}\,}_{2}}\]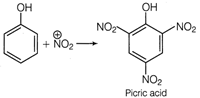 Molecular formula\[={{C}_{8}}{{H}_{3}}{{N}_{3}}{{O}_{7}}\] Degree of unsaturation = \[(C+1)-\frac{H}{2}+\frac{N}{2}\] \[=(6+1)-\frac{3}{2}+\frac{3}{2}=7-0=7\] Nature of compound The compound is acidic in nature due to presence of three strong electron withdrawing groups\[(N{{O}_{2}})\].
Molecular formula\[={{C}_{8}}{{H}_{3}}{{N}_{3}}{{O}_{7}}\] Degree of unsaturation = \[(C+1)-\frac{H}{2}+\frac{N}{2}\] \[=(6+1)-\frac{3}{2}+\frac{3}{2}=7-0=7\] Nature of compound The compound is acidic in nature due to presence of three strong electron withdrawing groups\[(N{{O}_{2}})\]. 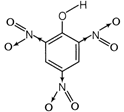
You need to login to perform this action.
You will be redirected in
3 sec
