A) Duprene
B) Natural rubber
C) Buna-S
D) Buna-N
Correct Answer: A
Solution :
Idea This problem contains conceptual mixing of molecular structure determination and polymerisation of identified monomer. To solve this problem students are advised to follow following steps. ? Calculate the value of degree of unsaturation. ? Draw the possible structures. ? Choose the correct structure among all possible structures. ? Then, complete chemical reaction according to given information. Determination of molecular structure of\[{{C}_{4}}{{H}_{5}}Cl\] Degree of unsaturation\[=(4+1)-\frac{6}{2}=5-3=2\] Since this molecule does not contain any ring or tripple bond. hence it must be a diene.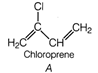 or
or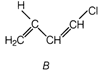 A is chloroprene when it undergoes polymerisation it produces duprene also known as neoprene.
A is chloroprene when it undergoes polymerisation it produces duprene also known as neoprene. 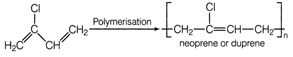 Chloroprene may undergo polymerisation via two modes either free radical polymerization and Ziegler natta polymerization. TEST Edge Student .are advised to go through the study of various polymerisation reaction and their characteristics properties such as nylon, bakelite etc.
Chloroprene may undergo polymerisation via two modes either free radical polymerization and Ziegler natta polymerization. TEST Edge Student .are advised to go through the study of various polymerisation reaction and their characteristics properties such as nylon, bakelite etc.
You need to login to perform this action.
You will be redirected in
3 sec
