A) 1
B) 2
C) 3
D) 4
Correct Answer: B
Solution :
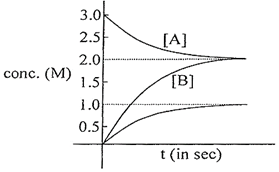
\[-\frac{d\left[ A \right]}{dt}=\frac{1}{x}\frac{d[B]}{dt}=\frac{1}{y}\frac{d[C]}{dt}\] \[-\frac{\left( 2-3 \right)}{t}=\frac{1}{x}\frac{\left( 2-0 \right)}{t}\Rightarrow x=2\] \[-\frac{\left( 2-3 \right)}{t}=\frac{1}{y}\frac{\left( 1-0 \right)}{t}\Rightarrow y=1\] \[\therefore \]\[{{K}_{C}}=\frac{{{[B]}^{x}}{{[C]}^{y}}}{[A]}=\frac{{{2}^{2}}\times {{1}^{1}}}{2}=2\]You need to login to perform this action.
You will be redirected in
3 sec
