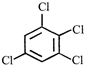
 is
is
A) \[2.86\,D\]
B) \[2.25\,\,D\]
C) \[1.5\,\,D\]
D) \[0\,\,D\]
Correct Answer: C
Solution :
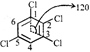 Dipole moments of \[2Cl\] and \[5Cl\]are vectorically cancelled. It is duel \[l\] \[Cl\] and \[3\,\,Cl\] \[{{\mu }^{2}}\] \[=\mu _{1}^{2}+\mu _{2}^{2}+2{{\mu }_{1}}{{\mu }_{2}}\cos \theta \] \[={{(1.5)}^{2}}+{{(1.5)}^{2}}+2\times 1.5\times 1.5\times 1.5\cos \,120\]\[\therefore \] \[\mu =1.5D\]
Dipole moments of \[2Cl\] and \[5Cl\]are vectorically cancelled. It is duel \[l\] \[Cl\] and \[3\,\,Cl\] \[{{\mu }^{2}}\] \[=\mu _{1}^{2}+\mu _{2}^{2}+2{{\mu }_{1}}{{\mu }_{2}}\cos \theta \] \[={{(1.5)}^{2}}+{{(1.5)}^{2}}+2\times 1.5\times 1.5\times 1.5\cos \,120\]\[\therefore \] \[\mu =1.5D\]
You need to login to perform this action.
You will be redirected in
3 sec
