A)
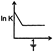
B)
![]()
C)
![]()
D)
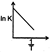
Correct Answer: D
Solution :
\[K=A{{e}^{-{{E}_{a}}/RT}}\] \[\ln \,K=\frac{-{{E}_{a}}}{RT}\,+\ln \,A\]You need to login to perform this action.
You will be redirected in
3 sec
