A) \[s{{p}^{2}},s{{p}^{3}},s{{p}^{3}}d,sp\]
B) \[s{{p}^{3}},s{{p}^{3}}d,s{{p}^{3}}d,sp\]
C) \[s{{p}^{3}},s{{p}^{3}}{{d}^{2}},s{{p}^{3}}d,s{{p}^{2}}\]
D) \[s{{p}^{2}},s{{p}^{3}}d,s{{p}^{3}}{{d}^{2}},s{{p}^{2}}\]
Correct Answer: B
Solution :
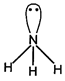 H.B. \[=\sigma \]bond + lone pair \[=3\sigma +1=4(s{{p}^{3}})\]
H.B. \[=\sigma \]bond + lone pair \[=3\sigma +1=4(s{{p}^{3}})\] 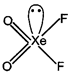 H.B.\[=\sigma \]bond + lone pair\[=4\sigma +1=5(s{{p}^{3}}d)\]
H.B.\[=\sigma \]bond + lone pair\[=4\sigma +1=5(s{{p}^{3}}d)\] 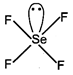 H.B. \[c=\frac{a}{m}\]bond + lone pair\[y=mx+c\]
H.B. \[c=\frac{a}{m}\]bond + lone pair\[y=mx+c\] You need to login to perform this action.
You will be redirected in
3 sec
