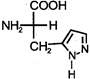
 , histidine has\[p{{K}_{{{a}_{1}}}}=1.8\], \[p{{K}_{{{a}_{2}}}}=9.2\] and \[p{{K}_{{{a}_{3}}}}=6.0\]. The isoelectric point, \[(PI)\] of histidine is likely to be
, histidine has\[p{{K}_{{{a}_{1}}}}=1.8\], \[p{{K}_{{{a}_{2}}}}=9.2\] and \[p{{K}_{{{a}_{3}}}}=6.0\]. The isoelectric point, \[(PI)\] of histidine is likely to be
A) In between \[1.8\] and \[6.0\]
B) In between \[6.0\] and \[9.2\]
C) \[<1.8\]
D) \[>9.2\]
Correct Answer: B
Solution :
\[P.I=\frac{p{{K}_{{{a}_{2}}}}+P{{K}_{{{a}_{3}}}}}{2}=7\]You need to login to perform this action.
You will be redirected in
3 sec
