| (I) | (II) 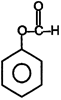 | (III) 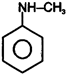 | (IV) 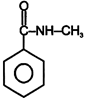 |
A) \[(II)>(III)>(IV)>(I)\]
B) \[(III)>(II)>(IV)>(I)\]
C) \[(IV)>(I)>(III)>(II)\]
D) \[(III)>(II)>(I)>(IV)\]
Correct Answer: B
Solution :
+ M group increases electron density and -M group decreases electron density in aromatic ring.You need to login to perform this action.
You will be redirected in
3 sec
