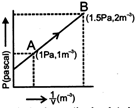
 Calculate the work done (in joule) in the process. [In 2 =0.7]
Calculate the work done (in joule) in the process. [In 2 =0.7]
A) 0.6 Joule
B) 6 Joule
C) 8 Joule
D) 0.8 Joule
Correct Answer: A
Solution :
\[p=m\times \frac{1}{V}\,+C\] \[1=m\times 1+C\] \[\,1.5=m\times 2+C\] \[m=0.5m\,C=0.5\] \[P=\frac{1}{2V}\,+\frac{1}{2}\] Work done \[=-\int_{{}}^{{}}{PdV}\] \[=-\int_{1}^{0.5}{\frac{1}{2}\,\left( \frac{1}{V}+1 \right)dv=0.6}\] JouleYou need to login to perform this action.
You will be redirected in
3 sec
