A) \[0.072M\,{{\min }^{-1}}\]
B) \[0.036M\,{{\min }^{-1}}\]
C) \[0.1296M\,{{\min }^{-1}}\]
D) \[1M\,{{\min }^{-1}}\]
Correct Answer: A
Solution :
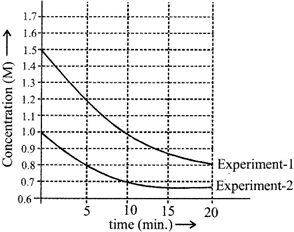
Rate law \[r=k{{[A]}^{m}}\] \[{{r}_{1}}=\left( \frac{0.3}{5} \right)M/\min \] min from graph (exp. 1) \[{{r}_{2}}=\left( \frac{0.2}{5} \right)M/\min \] from graph (exp. 2) \[\frac{{{r}_{1}}}{{{r}_{2}}}=\frac{3}{2}={{\left\{ \frac{{{[A]}_{1}}}{{{[A]}_{2}}} \right\}}^{m}}={{\left( \frac{1.5}{1} \right)}^{m}}\Rightarrow m=1\] \[\frac{0.3}{5}=k(1.5)\Rightarrow k=\frac{1}{25}{{\min }^{-1}}\] \[r=k\times [A]\Rightarrow \frac{1}{25}\times 1.8=0.072\,M\,{{\min }^{-1}}\]You need to login to perform this action.
You will be redirected in
3 sec
