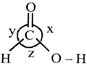
A) \[x>y>z\]
B) \[x<y<z\]
C) \[x=y>z\]
D) Can't be predicted
Correct Answer: A
Solution :
 Therefore, repulsion between both \[C-O\] bonds will be greater than the repulsion between \[C-H\] bond and \[C-OH\] bond or between \[C-H\] and \[C=O\] bond. Repulsion between \[C-H\] and C = 0 bond is greater than that of between \[C-H\] and \[C-OH\]bond, because double bond character in \[C-OH\] bond will always be less than \[C=O\].
Therefore, repulsion between both \[C-O\] bonds will be greater than the repulsion between \[C-H\] bond and \[C-OH\] bond or between \[C-H\] and \[C=O\] bond. Repulsion between \[C-H\] and C = 0 bond is greater than that of between \[C-H\] and \[C-OH\]bond, because double bond character in \[C-OH\] bond will always be less than \[C=O\].
You need to login to perform this action.
You will be redirected in
3 sec
