A) \[C=0\]
B) \[C={{C}_{v}}\]
C) \[C>{{C}_{v}}\]
D) \[C<{{C}_{v}}\]
Correct Answer: C
Solution :
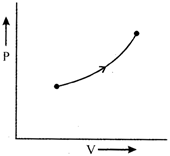
According to graph, pressure is increasing, also volume is increasing hence T also increases. It means that energy is used both in increasing internal energy and work done \[nCdt=n{{C}_{v}}dt+P\Delta V\] \[\therefore \]\[C>{{C}_{v}}\]You need to login to perform this action.
You will be redirected in
3 sec
