A) 2 two - centre - two - electron bonds and 4 three - centre - three - electron bonds.
B) 4 two - centre - two - electron bonds and 2 three - centre - three - electron bonds
C) 3 two - centre - two - electron bonds and 3 three - centre - three - electron bonds.
D) all six identical bonds.
Correct Answer: B
Solution :
Structure of \[{{B}_{2}}{{H}_{6}}\] is as follows: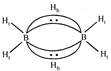 or
or 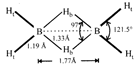 Thus the diborane molecule has four two-centre-two- electron bonds (2c-2e bonds) also called usual bonds and two three-centre-two-electron bonds (3c?2e) also called banana bonds. Hydrogen attached to usual and banana bonds are called \[{{\text{H}}_{\text{t}}}\] (terminal H) and\[{{\text{H}}_{\text{b}}}\](bridged H) respectively.
Thus the diborane molecule has four two-centre-two- electron bonds (2c-2e bonds) also called usual bonds and two three-centre-two-electron bonds (3c?2e) also called banana bonds. Hydrogen attached to usual and banana bonds are called \[{{\text{H}}_{\text{t}}}\] (terminal H) and\[{{\text{H}}_{\text{b}}}\](bridged H) respectively.
You need to login to perform this action.
You will be redirected in
3 sec
