A) Hofmann-bromamide reaction
B) Schmidt reaction
C) Curtius reaction
D) Beckmann reaction.
Correct Answer: A
Solution :
\[R-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-N{{H}_{2}}+B{{r}_{2}}\to R-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-\overset{..}{\mathop{N}}\,HBr\xrightarrow[-{{H}^{+}}]{{}}\]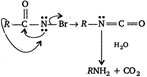 This reaction is called Hofmann-bromamide reaction.
This reaction is called Hofmann-bromamide reaction.
You need to login to perform this action.
You will be redirected in
3 sec
