A) \[15R\theta \]
B) \[\frac{3}{8}R\theta \]
C) \[\frac{2}{15}{{R}^{2}}\theta \]
D) \[\frac{2}{15}R\theta \]
Correct Answer: A
Solution :
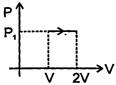
Process is isobaric. So \[dQ=n{{C}_{p}}dT\] For monoatomic gas, \[{{C}_{\upsilon }}=\frac{3}{2}R\] and \[{{C}_{p}}=\frac{5}{2}\operatorname{R}\] So, \[dQ=2\times \frac{5}{2}R\times (4\theta -\theta )=15R\theta \]You need to login to perform this action.
You will be redirected in
3 sec
