A) Heating \[{{\operatorname{AlCl}}_{3}}\].\[6{{H}_{2}}O\]
B) By passing dry HCI over hot aliminium powder
C) By passing dry \[C{{l}_{2}}\] over hot aliminium powder
D) By passing dry\[C{{l}_{2}}\] over a hot mixture of alumina and coke
Correct Answer: A
Solution :
| \[2Al+6HCl\xrightarrow{\Delta ,\operatorname{air}}2{{\operatorname{AlCl}}_{3}}+3{{H}_{2}}\] |
| \[2Al+3C{{l}_{2}}\xrightarrow{\Delta }2AlC{{l}_{3}}\] \[{{\operatorname{Al}}_{2}}{{O}_{3}}+3C+3C{{l}_{2}}\xrightarrow{1000{}^\circ C}2AlC{{l}_{3}}+3CO\] |
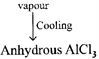 |
| \[{{\operatorname{AlCl}}_{3.}}6{{H}_{2}}O\xrightarrow{\Delta }Al{{\left( OH \right)}_{3}}+3HCl+3{{H}_{2}}O\] |
| Thus \[{{\operatorname{AlCl}}_{3}}\] cannot be obtained by heating \[{{\operatorname{AlCl}}_{3}}\]\[6{{H}_{2}}O\] |
You need to login to perform this action.
You will be redirected in
3 sec
