A) \[{{H}_{3}}P{{O}_{3}}\] is stronger acid than\[{{H}_{3}}P{{O}_{4}}\]
B) \[{{H}_{3}}P{{O}_{3}}\] is dibasic and reducing.
C) \[{{H}_{3}}P{{O}_{4}}\] tribasic and reducing
D) [a] and [b] both
Correct Answer: D
Solution :
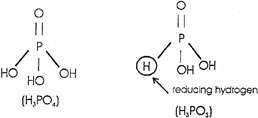 Strength of phosphorus oxy acid depends upon the number of OH groups per\[P=O\]group. It is the \[P=O\]group which induces polarisation and helps in the release of proton from-OH group. \[{{H}_{3}}P{{O}_{3}}>{{H}_{3}}{{P}_{4}}\]
Strength of phosphorus oxy acid depends upon the number of OH groups per\[P=O\]group. It is the \[P=O\]group which induces polarisation and helps in the release of proton from-OH group. \[{{H}_{3}}P{{O}_{3}}>{{H}_{3}}{{P}_{4}}\]
You need to login to perform this action.
You will be redirected in
3 sec
