| Adsorption of a gas follows Freundlich adsorption isotherm. In the given plot, x is the mass of the gas y adsorbed on mass m of the adsorbent at pressure p.\[\frac{x}{m}\] |
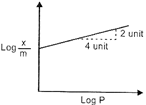 |
A) \[{{\text{P}}^{\frac{1}{4}}}\]
B) \[{{\text{P}}^{2}}\]
C) P
D) \[{{\text{P}}^{\frac{1}{2}}}\]
Correct Answer: D
Solution :
| \[\frac{x}{m}\,=\,K\,\times \,{{p}^{l/n}}\] |
| \[\log \,\frac{x}{m}\,=\,\log \,\text{K}\,+\frac{1}{n}\log \text{P}\] |
| \[m\,=\,\frac{1}{n}\,=\,\frac{2}{4}\,=\,\frac{1}{2}\,\]\[\Rightarrow \]\[n=2\] |
| So, \[\frac{x}{m}\,=\,\text{K}\times {{\text{p}}^{\text{1/2}}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
