| In the reaction : \[A+2B\xrightarrow{{}}\text{product}\] |
| \[\text{rate}=k\,[A]\,\,{{[B]}^{0}}\] |
| The initial concentration of both A and Bare a M. The graph of concentration of B vs time is: \[({{t}_{1}}={{t}_{{\scriptstyle{}^{1}/{}_{2}}}}\,\text{for}\,\text{A)}\] |
A)
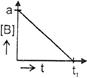
B)
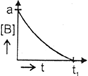
C)
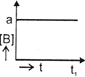
D)
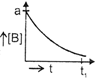
Correct Answer: B
Solution :
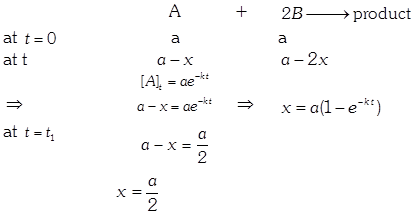
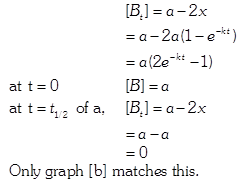
You need to login to perform this action.
You will be redirected in
3 sec
