A) The coordination number of each type of ion in \[CsCl\] crystal is 8.
B) A metal that crystallises in bcc structure has a coordination number of 12.
C) A unit cell of an ionic crystal shares some of its ions with other unit cells.
D) The length of the unit cell in \[NaCl\] is 276 pm.
Correct Answer: B , D
Solution :
| [a] The unit cell of \[CsCl\]has bcc arrangement of ions in which each ion has eight oppositely charged ions around it, in the nearest neighbours as shown below : |
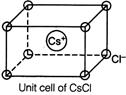 |
| [b] In bcc, coordination number of metal is 8. |
| [c] In an unit cell, a comer is shared in eight unit cells and a face centre is shared between two adjacent unit cells. |
| [d] In \[NaCl\] unit cell; \[2\,({{r}_{N{{a}^{+}}}}+{{r}_{C{{l}^{-}}}})=a\] |
| \[\Rightarrow \]\[a=2\,(95+181)=552\,pm\] |
| Thus, incorrect statements in given options [b] and [d]. |
Solution :
Same as aboveYou need to login to perform this action.
You will be redirected in
3 sec
