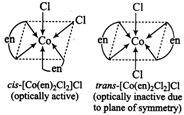
A) \[cis-[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
B) \[trans-[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
C) \[cis-[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
D) \[trans-[Co{{(N{{H}_{3}})}_{4}}C{{l}_{3}}]\]
Correct Answer: A
Solution :
| Optical isomerism arises in those compounds where the mirror images are non-superimposable. |
| Complexes like \[[\,M{{(AA)}_{2}}{{X}_{2}}]\] (only c is form) and \[[M{{(AA)}_{3}}]\]exhibit optical isomerism. |
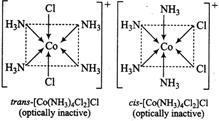
 \[[Co(N{{H}_{3}})C{{l}_{2}}]Cl\]can exist in both c is and trans forms that are given below: \[[Co(N{{H}_{3}})C{{l}_{2}}]Cl\]can exist in both c is and trans forms that are given below: |
 |
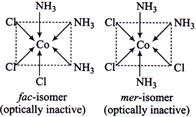
| \[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{3}}]\]exists in fac and mer-isomeric forms and both are optically inactive. |
 |
| Thus, the correct option is [a]. |
You need to login to perform this action.
You will be redirected in
3 sec
