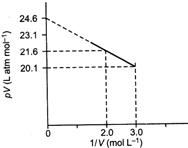
| For one mole of a van der Waals' gas when \[b=0\]and T = 300 K, the pV vs 1/V plot is shown below. The value of the van der Waals' constant a \[(\text{atm}\,\text{Lmo}{{\text{l}}^{-2}})\] |
 |
A) 1.0
B) 4.5
C) 1.5
D) 3.0
Correct Answer: C
Solution :
| The van der Waals' equation of state is |
| \[\left( p+\frac{{{n}^{2}}a}{{{V}^{2}}} \right)(V-nb)=nRT\] |
| For one mole and when 6=0, the above equation condenses to |
| \[\left( p+\frac{a}{{{V}^{2}}} \right)V=RT\] |
| \[\Rightarrow \] \[pV=RT-\frac{a}{V}\] | ? (i) |
| Eq. (i) is a straight line equation between | |
| pV and \[\frac{1}{V}\]whose slope is \['-a'.\]Equating with slope of the straight line given in the graph. | |
| \[-a=\frac{20.1-21.6}{3-2}=-1.5\] | |
| \[\Rightarrow \] \[a=1.5\] | |
You need to login to perform this action.
You will be redirected in
3 sec
