| A gas can be taken from A to B via two different processes ACB and ADB. |
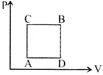 |
| When the 'path ACB is used 60 J if heat flows into the system and 30 J of work is done by the system. If path ADB is used work done by the system is 10 J. The heat flows into the system in path ADB is: |
A) 80 J
B) 20 J
C) 100 J
D) 40 J
Correct Answer: D
Solution :
 |
| \[\Delta {{\text{Q}}_{\text{ACB}}}=\Delta {{\text{W}}_{\text{ACB}}}+\Delta {{\text{U}}_{\text{ACB}}}\] |
| \[\Rightarrow \] \[60J=30J+\Delta {{U}_{ABC}}\] |
| \[\Rightarrow \] \[\Delta {{\text{U}}_{\text{ABC}}}=30\,\text{J}\] |
| \[\Rightarrow \] \[\Delta {{U}_{ADB}}=\Delta {{U}_{ACB}}=\,30\,J\] |
| \[\Delta {{Q}_{ACD}}=\Delta {{U}_{ACB}}+\Delta {{W}_{ADB}}\] |
| \[=\,10\,\,J+30\,J=\,40\,J.\] |
You need to login to perform this action.
You will be redirected in
3 sec
