| A container is separated into two chambers A and B of equal volumes by a conducting wall. |
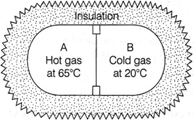 |
| Heat cannot leave or enters the chambers A and B from outer boundary except through partition AB. Compartment A is filled with hot gas and compartment B is filled with same cold gas in equal amount. Hot gas cool down from \[65{}^\circ C\] to \[63{}^\circ C,\]choose correct, |
A) entropy change of A is more
B) entropy change of B is more
C) entropy change of A and B is same
D) total entropy change of A and B is negative
Correct Answer: B
Solution :
| Heat lost by hot gas in A \[=-\Delta Q=-m{{C}_{V}}\Delta T\] |
| Heat gained by cold gas in B \[=+\,\Delta Q=m{{C}_{V}}\Delta T\] |
| Entropy change for hot gas in A is \[{{S}_{A}}=\frac{-\Delta Q}{{{T}_{A}}}=\frac{-\Delta Q}{273+65}\] |
| Entropy change for cold gas in B is \[{{S}_{B}}=\frac{+\Delta Q}{273+20}\] |
| Clearly, \[\left| {{S}_{B}} \right|>\left| {{S}_{A}} \right|.\] |
| So, more entropy gain occurs in B than entropy loss in A. Total entropy of universe increases due to this process. |
You need to login to perform this action.
You will be redirected in
3 sec
