A) 7.90 kJ \[{{\text{K}}^{-1}}\]\[\text{k}{{\text{g}}^{-1}}\]
B) 2.64 kJ \[{{\text{K}}^{-1}}\]\[\text{k}{{\text{g}}^{-1}}\]
C) 8.49 kJ \[{{\text{K}}^{-1}}\] \[\text{k}{{\text{g}}^{-1}}\]
D) 9.26 kJ \[{{\text{K}}^{-1}}\]\[\text{k}{{\text{g}}^{-1}}\]
Correct Answer: D
Solution :
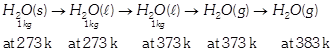 \[\Delta S=\Delta {{S}_{1}}+\Delta {{S}_{2}}+\Delta {{S}_{3}}+\Delta {{S}_{4}}\] \[=\frac{334}{273}+4.2\ell n\frac{373}{273}+\frac{2491}{373}+2\ell n\frac{383}{373}\] \[=9.267kJ\,k{{g}^{-\,1}}{{K}^{-\,1}}.\]
\[\Delta S=\Delta {{S}_{1}}+\Delta {{S}_{2}}+\Delta {{S}_{3}}+\Delta {{S}_{4}}\] \[=\frac{334}{273}+4.2\ell n\frac{373}{273}+\frac{2491}{373}+2\ell n\frac{383}{373}\] \[=9.267kJ\,k{{g}^{-\,1}}{{K}^{-\,1}}.\]
You need to login to perform this action.
You will be redirected in
3 sec
