| A gas is contained in a small container which is connected to a vacuum chamber via a stop value. |
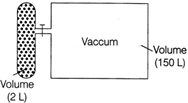 |
| The 2L container contains 5 moles of gas at 300 K. Given, In\[76=4.3\]. At time \[t=0,\]stop value is opened and gas expands in 150 L vacuum chamber. |
| Now, Choose the correct option. |
A) Due to expansion, temperature of gas falls
B) Due to expansion, 54 kJ of energy becomes unavailable
C) Due to expansion, gas does 54 kJ of work
D) Internal energy changes by 54 kJ due to expansion process
Correct Answer: B
Solution :
| As gas expands in vacuum, work done by gas is zero. |
| So, \[\Delta U=0\] and temperature of gas remains constant. |
| Unavailable energy is |
| \[E=T\Delta S=nRT\]In \[\left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\] |
| \[=5\times 8.314\times 300\times \ln \left( \frac{152}{5} \right)\]\[=5\times 8.314\times 300\times 4.3\approx 54\,kJ\] |
You need to login to perform this action.
You will be redirected in
3 sec
