A)
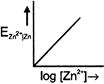
B)
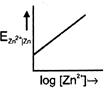
C)
D)
Correct Answer: B
Solution :
\[{{E}_{Z{{n}^{2+}}|Zn}}=E{{{}^\circ }_{Z{{n}^{2+}}|Zn}}+\frac{0.059}{2}\log \,[Z{{n}^{+\,2}}]\] Comparing with \[y=mx+c,\] graph between \[{{E}_{Z{{n}^{2+}}|Zn}}\]and \[\log \,[Z{{n}^{2\,+}}]\] is a straight line with positive slope and positive intercept.You need to login to perform this action.
You will be redirected in
3 sec
