| Graph between \[log\text{ }K\] and \[\frac{1}{T}\] [Where K is rate constant \[\left( {{S}^{^{-1}}} \right)\] and T is temperature (K)] is a straight line with \[OX=5,\,\theta ={{\tan }^{-1}}\left[ -\frac{1}{2.303} \right]\] |
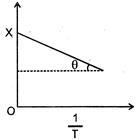 |
| Hence \[{{E}_{a}}\] and log A respectively will be: |
A) \[2.303\times 2\,cal,\,5\]
B) \[\frac{2}{3.303}cal,\,{{e}^{5}}\]
C) \[2\,cal,\,5\]
D) None of these
Correct Answer: C
Solution :
| \[K=A{{e}^{-\,\frac{{{E}_{a}}}{R\,T}}}\] |
| \[\log K=\log A-\frac{{{E}_{a}}}{2.303\,R}\times \frac{1}{T}\] |
| Slope \[=\tan \theta =\left[ -\frac{1}{2.303} \right]=-\frac{{{E}_{a}}}{2.303\,R}\] |
| so \[{{E}_{a}}=R=2\,\,cal/mol\] |
You need to login to perform this action.
You will be redirected in
3 sec
