A) \[PO_{4}^{3-}\]
B) \[NO_{2}^{-}\]
C) \[NO_{3}^{-}\]
D) \[CO_{3}^{2-}\]
Correct Answer: A
Solution :
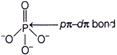
| \[p\pi -d\pi \] overlapping is formed when p-orbital of one atom and d-orbital of another atom overlap laterally. |
| In case of \[PO_{4}^{3-},P\] is \[s{{p}^{3}}\text{-}\]hybridised, it forms four P-O \[\sigma \] bonds by overlap of \[s{{p}^{3}}\text{-}\]hybridised orbitals of P with p-orbitals of O. The bond is however formed by overlap of p-orbitals of O and d-orbitals of P. N and C on the other hand cannot form \[p\pi -d\pi \] bond, since they do not have d-orbitals. |
 |
You need to login to perform this action.
You will be redirected in
3 sec
