A) \[ds{{p}^{2}},\] square planar
B) \[s{{p}^{3}}{{d}^{2}},\] octahedral
C) \[s{{p}^{3}},\] tetrahedral
D) \[s{{p}^{3}}{{d}^{2}},\] square planar
Correct Answer: D
Solution :
[d]| The hybridisation of a compound can be calculated using formula. |
| \[H=\frac{1}{2}[V+MO\pm \text{anion}/\text{cation}]\] |
| where, H= Hybridised orbitals |
| V= Valence |
| MO = Number of monoatomic atoms |
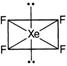
| For \[Xe{{F}_{4}}\] |
| \[H=\frac{1}{2}[8+4]=6\] |
| \[\Rightarrow \] \[s{{p}^{3}}{{d}^{2}}\] |
| It has 4 bond pairs and 2 lone pair, where 2 lone pair occupies axial positions and four F occupies equatorial positions. So, its geometry is square planar and not octahedral. |
 |
You need to login to perform this action.
You will be redirected in
3 sec
