A)
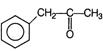
B)
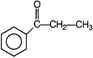
C)
![]()
D)
![]()
Correct Answer: A
Solution :
[a]| The organic compound with molecular formula \[{{C}_{9}}{{H}_{10}}O\] forms an orange-red ppt. with 2, 4-DNP reagent and gives yellow ppt. on heating with \[{{I}_{2}}\] and NaOH. This confirms the presence of carboxyl group in compound . Also, it does not reduces Tollen's reagent or Fehling's solution, this shows it is a kenotic group and not an aldehyde. As it does not decolourises bromine water it indicates the absence of unsaturated bonds. The reactions are as follows: |
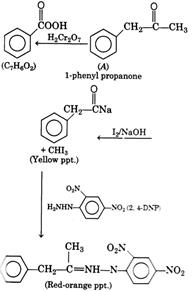 |
You need to login to perform this action.
You will be redirected in
3 sec
