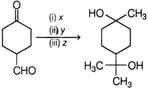
A) \[{{[Ag\,(N{{H}_{3}})]}^{2+}}O{{H}^{-}},{{H}^{+}},C{{H}_{3}}OH,C{{H}_{3}}MgBr\]
B) \[C{{H}_{3}}MgBr,{{H}^{+}}{{]}^{+}}C{{H}_{3}}OH,[Ag\,(N{{H}_{3}})_{2}^{+}O{{H}^{-}}\]
C) \[C{{H}_{3}}MgBr,{{[Ag\,{{(N{{H}_{3}})}_{2}}]}^{+}}O{{H}^{-}},\,\,{{H}^{+}}\text{/}C{{H}_{3}}OH\]
D) \[Ag\,{{(N{{H}_{3}})}_{2}}{{]}^{+}}O{{H}^{-}},C{{H}_{3}}MgBr,\,\,{{H}^{+}}|C{{H}_{3}}OH\]
Correct Answer: A
Solution :
[a] 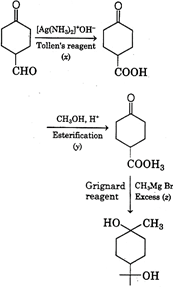 |
| Reagent \[x\,{{[Ag\,{{(N{{H}_{3}})}_{2}}]}^{+}}O{{H}^{-}}\] is a Tollen's reagent. It will only oxidises aldehyde group and not ketone group. Reagent y, \[C{{H}_{3}}OH\]is an alcohol, which then reacts with formed carboxylic acid to give ester. |
| In presence of reagent z, \[C{{H}_{3}}MgBr\](excess) it will reduce both ketone and ester group to alcoholic group. |
You need to login to perform this action.
You will be redirected in
3 sec
