| In the reaction, |
| \[P+Q\xrightarrow{{}}R+S\] |
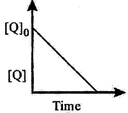
| The time taken for \[75%\] reaction of P is twice the time taken for \[50%\] reaction of P. The concentration of Q varies with reaction time as shown in the figure. The overall order of the reaction is |
 |
A) 2
B) 3
C) 0
D) 1
Correct Answer: D
Solution :
| For P, if \[{{\operatorname{t}}_{50%}}=x\]then\[{{t}_{75%}}=2\operatorname{x}\] |
| This is true only for first order reaction. |
| So, order with respect to P is 1. |
| Further the graph shows that concentration of Q decreases with time. So rate, with respect to Q, remains constant. Hence, it is zero order wrt Q. |
| So, overall order is \[1+0=1\] |
You need to login to perform this action.
You will be redirected in
3 sec
