A) 1.6
B) 0.25
C) 0.4
D) 0.16
Correct Answer: A
Solution :
| Given, \[\alpha \]for \[\operatorname{HI}=0.8\] |
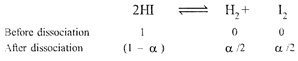 |
| \[\therefore \]\[{{K}_{e}}~=\frac{{{\alpha }^{2}}}{4{{(1-\alpha )}^{2}}}=\frac{{{(0.8)}^{2}}}{4{{(1-0.8)}^{2}}}=4\] |
| Now |
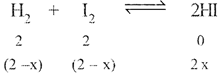 |
| \[\therefore K_{e}^{'}=\frac{1}{{{K}_{e}}}=\frac{4{{x}^{2}}}{{{\left( 2-x \right)}^{2}}}=\frac{1}{4}\] |
| \[\operatorname{x}=2/5\] |
| Thus, \[{{\operatorname{I}}_{2}}\]left =\[2-\frac{2}{5}=\frac{8}{5}\]mole \[=\frac{8}{5}\times 2\]equivalent |
| Or \[{{\operatorname{Na}}_{2}}{{S}_{2}}{{O}_{3}}\]required can be calculate as; |
| \[\operatorname{Meq}.\,Of\,N{{a}_{2}}{{S}_{2}}{{O}_{3}}=-Meq.Of\,{{I}_{2}}\,left\] |
| \[2\times V=\frac{8}{5}\times 2\times 1000\] |
| \[\therefore \]\[V=1600mL=1.6\,litre\] |
You need to login to perform this action.
You will be redirected in
3 sec
