| A system undergoes a cycle consisting of three processes. | ||||||||||||||||
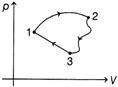 | ||||||||||||||||
| Process involved are listed as, | ||||||||||||||||
|
A) \[200\text{ }kJ,\text{ }50\text{ }kJ,\text{ }100\text{ }kJ,\text{ }300\text{ }kJ\]
B) \[200\text{ }kJ,\text{ }50\text{ }kJ,\text{ }300\text{ }kJ,\text{ }100\text{ }kJ\]
C) \[50\text{ }kJ,\text{ }100\text{ }kJ,\text{ }200\text{ }kJ,\text{ }300\text{ }kJ\]
D) \[300\text{ }kJ,\text{ }200\text{ }kJ,\text{ }200\text{ }kJ,\text{ }50\text{ }kJ\]
Correct Answer: A
Solution :
| From first law of thermodynamics, |
| we have, \[\Delta Q-\Delta W=\Delta E\] |
| Applied to process \[1\to 2\] we get |
| \[a-100=100\Rightarrow a=200KJ\] |
| For process \[3\to 1\], |
| \[100-d=-200\] |
| \[d=300KJ\] |
| For entire cycle, \[\sum Q=\sum W\]\[\Rightarrow \,\,\,200+b+100=350\Rightarrow b=50KJ\] |
| For a cyclic process, \[\sum \Delta U=0\]\[\Rightarrow 100+c-200=0\]\[\Rightarrow \,\,\,\,\,\,\,\,\,\,c=100KJ\] |
You need to login to perform this action.
You will be redirected in
3 sec
