A) \[{{H}_{2}}S{{O}_{4}}>{{H}_{2}}S{{O}_{3}}>{{H}_{2}}{{S}_{2}}{{O}_{8}}\]
B) \[{{H}_{2}}{{S}_{2}}{{O}_{8}}>{{H}_{2}}S{{O}_{3}}>{{H}_{2}}S{{O}_{4}}\]
C) \[{{H}_{2}}{{S}_{2}}{{O}_{8}}>{{H}_{2}}S{{O}_{4}}>{{H}_{2}}S{{O}_{3}}\]
D) \[{{H}_{2}}S{{O}_{3}}>{{H}_{2}}{{S}_{2}}{{O}_{8}}>{{H}_{2}}S{{O}_{4}}\]
Correct Answer: C
Solution :
| The structures of are as follows: |
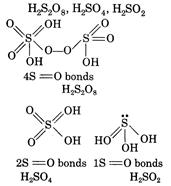 |
| Thus, the correct order of decreasing number of \[S=O\]bonds is |
| \[{{H}_{2}}{{S}_{2}}{{O}_{8}}>{{H}_{2}}S{{O}_{4}}>{{H}_{2}}S{{O}_{3}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
