A) Triplet intermediates can show syn addition whereas singlet intermediates can also show the ant addition
B) State of minimum multiplicity is most stable state due to least energy
C) The paired electronic configuration provides maximum multiplicity, which leads to more stabilization for intermediate
D) The electron is separate orbitals bring the state of maximum multiplicity according to formula 2S + 1, where 5 = sum of spin quantum numbers for all the electrons. This leads to net decrease in energy according to hunds rule of maximum multiplicity
Correct Answer: D
Solution :
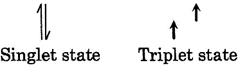 In triplet state electron exist in different orbital so \[e-{{e}^{-}}\] repulsion is less in triplet carbine or nitrene that is why triplet is more stable than singlet.
In triplet state electron exist in different orbital so \[e-{{e}^{-}}\] repulsion is less in triplet carbine or nitrene that is why triplet is more stable than singlet.
You need to login to perform this action.
You will be redirected in
3 sec
