| In which of the following reaction backward reaction is favoured. |
| I. \[H-C\equiv C-H+\overset{\oplus }{\mathop{N}}\,a\overset{\Theta }{\mathop{C}}\,{{H}_{3}}C{{H}_{4}}+H-C\equiv \overset{\Theta }{\mathop{C}}\,\overset{\oplus }{\mathop{N}}\,a\]II. \[C{{H}_{3}}\equiv \underset{\underset{O}{\mathop{\parallel }}\,}{\mathop{CO}}\,-H+\overset{\oplus }{\mathop{N}}\,a\overset{\Theta }{\mathop{O}}\,C{{H}_{3}}\]\[C{{H}_{3}}\equiv \underset{\underset{O}{\mathop{\parallel }}\,}{\mathop{C}}\,-\overset{\Theta }{\mathop{O}}\,\overset{\oplus }{\mathop{N}}\,a+C{{H}_{3}}OH\] |
III. 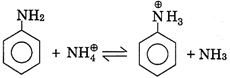 |
IV. 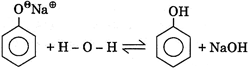 |
V. 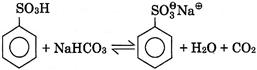 |
VI. 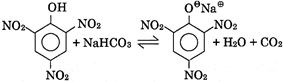 |
A) I, II, III, IV only
B) III, IV, V only
C) III, VI only
D) III, IV only
Correct Answer: D
Solution :
| Acidic strength order |
 |
| Acid Base reaction occur more stronger acid & more stronger base side to less stronger acid & less stronger base |
You need to login to perform this action.
You will be redirected in
3 sec
