| Given | ||
(I) 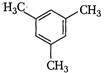 | (II) 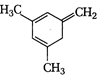 | (III) 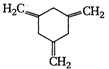 |
| The enthalpy of hydrogenation of these compounds will be the order as: | ||
A) \[I>II>III\]
B) \[III>II>I\]
C) \[II>III>I\]
D) \[II>I>III\]
Correct Answer: B
Solution :
| The enthalpy of hydrogenation of given compounds is inversely proportional to stability of alkene. |
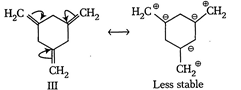 |
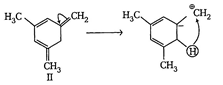 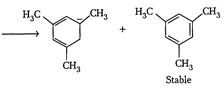 |
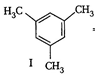 =Already aromatic compound because of \[6\pi {{e}^{-}}s\]Hence, correct order of enthalpy of hydrogenation is III>II>I =Already aromatic compound because of \[6\pi {{e}^{-}}s\]Hence, correct order of enthalpy of hydrogenation is III>II>I |
You need to login to perform this action.
You will be redirected in
3 sec
